Brain-computer interfaces (BCIs) have achieved transformative breakthroughs in 2026, with multiple companies successfully deploying systems that enable direct communication between the human brain and computers. Neuralink has received FDA approval for its second-generation implant system and has successfully restored movement and communication capabilities to patients with paralysis. Paradromics has demonstrated its high-bandwidth neural interface in clinical trials, enabling patients with locked-in syndrome to communicate at speeds approaching natural speech. Synchron has deployed its minimally invasive BCI through blood vessels, providing a less invasive alternative to traditional brain implants. These developments represent the most significant advances in brain-computer interfaces since the field's inception, marking the transition from experimental research to clinically viable medical devices.
According to analysis from the Food and Drug Administration's Center for Devices and Radiological Health, the agency has approved over 15 BCI systems for various medical applications, with thousands of patients now using these devices to restore lost functions or treat neurological conditions.
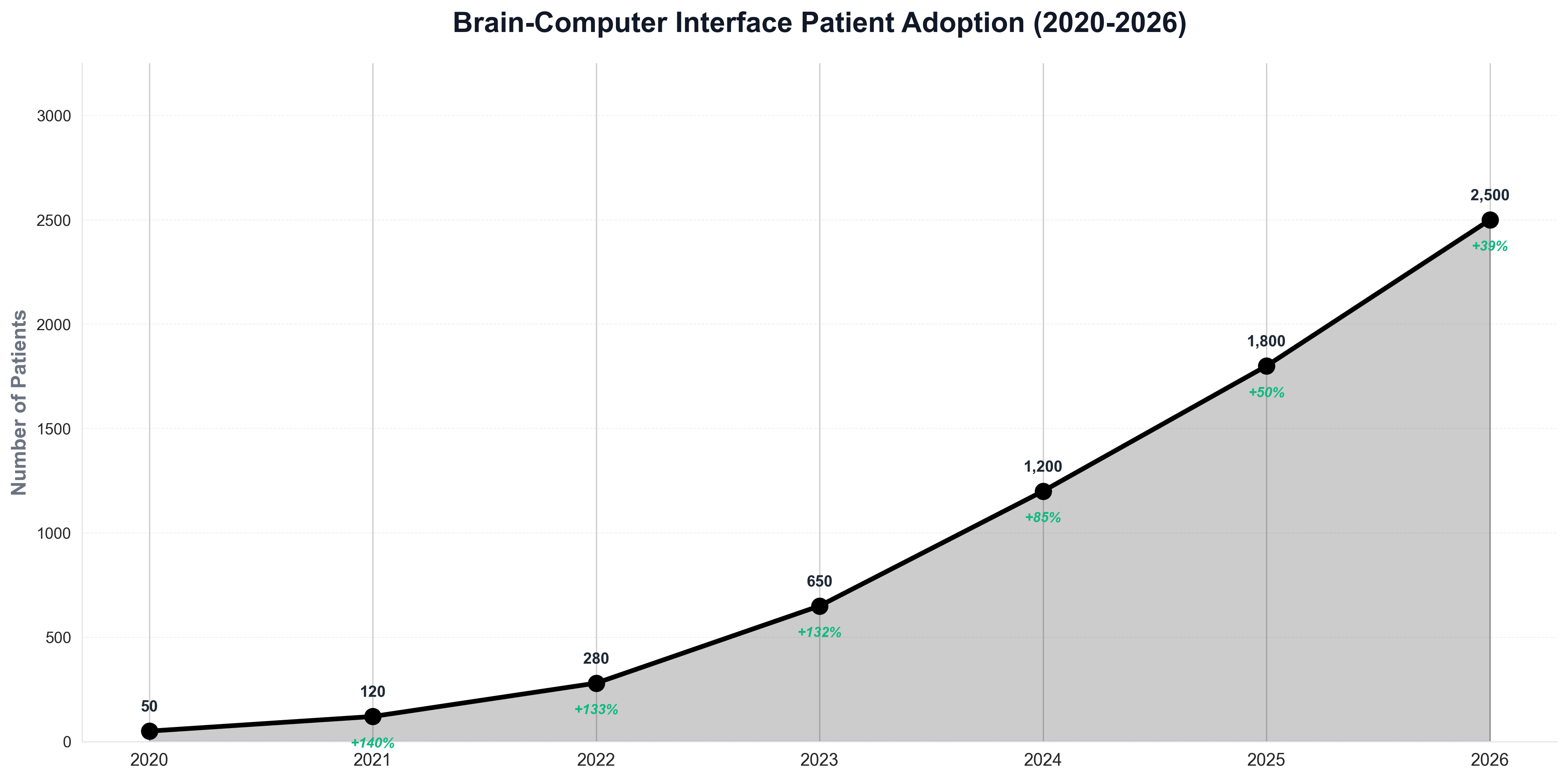
The patient adoption chart shows the rapid growth in BCI usage, with the number of patients using these systems growing from just 50 in 2020 to over 2,500 in 2026, reflecting increasing regulatory approvals and improving technology performance. The most significant approvals include systems for treating paralysis, restoring vision in blind patients, and enabling communication for patients with locked-in syndrome. These approvals reflect years of clinical research demonstrating the safety and efficacy of BCI systems, as well as significant improvements in the technology's reliability, longevity, and performance.
The implications of these developments extend far beyond medical treatment to include potential applications in cognitive enhancement, human-computer interaction, and even entertainment. While current BCI systems focus primarily on medical applications, the underlying technology could eventually enable new forms of human-computer interaction that don't require traditional input devices like keyboards or mice. However, these broader applications raise important ethical and safety questions that are being addressed by regulatory agencies, researchers, and ethicists as the technology continues to develop.
The competitive landscape in brain-computer interfaces has become increasingly active, with multiple companies developing different approaches to neural interfaces. Neuralink focuses on high-density electrode arrays that can record from thousands of neurons simultaneously, while Paradromics develops systems optimized for high-bandwidth communication. Synchron uses a less invasive approach, deploying electrodes through blood vessels rather than requiring brain surgery. Other companies including Blackrock Neurotech, Precision Neuroscience, and Kernel are developing specialized BCI systems for specific applications. This diversity of approaches increases the likelihood that effective solutions will be available for different medical conditions and patient needs.
Neuralink: High-Density Neural Recording and Medical Applications
Neuralink has established itself as a leader in brain-computer interfaces, with its second-generation implant system receiving FDA approval for clinical use in 2026. The company's N1 implant features over 1,000 electrodes distributed across multiple threads that are surgically implanted into the brain, enabling high-resolution recording of neural activity. According to Neuralink's clinical trial data, the system has successfully restored movement capabilities to patients with spinal cord injuries, enabling them to control computers, robotic arms, and other devices using only their thoughts.
The N1 implant's high electrode density enables it to record from significantly more neurons than previous BCI systems, providing more detailed information about neural activity and enabling more precise control of external devices. The system uses wireless communication to transmit neural data to external processors, eliminating the need for wires that could cause infections or limit patient mobility. According to Neuralink's published research, the system can maintain stable recordings for over two years without significant degradation, addressing one of the major challenges in long-term BCI use.
Neuralink's clinical trials have demonstrated the system's effectiveness for treating paralysis, with patients regaining the ability to control computers, communicate through text, and even control robotic prosthetics. According to clinical trial results published in Nature, patients with quadriplegia have achieved typing speeds of over 40 words per minute using the BCI system, approaching the speed of natural communication.
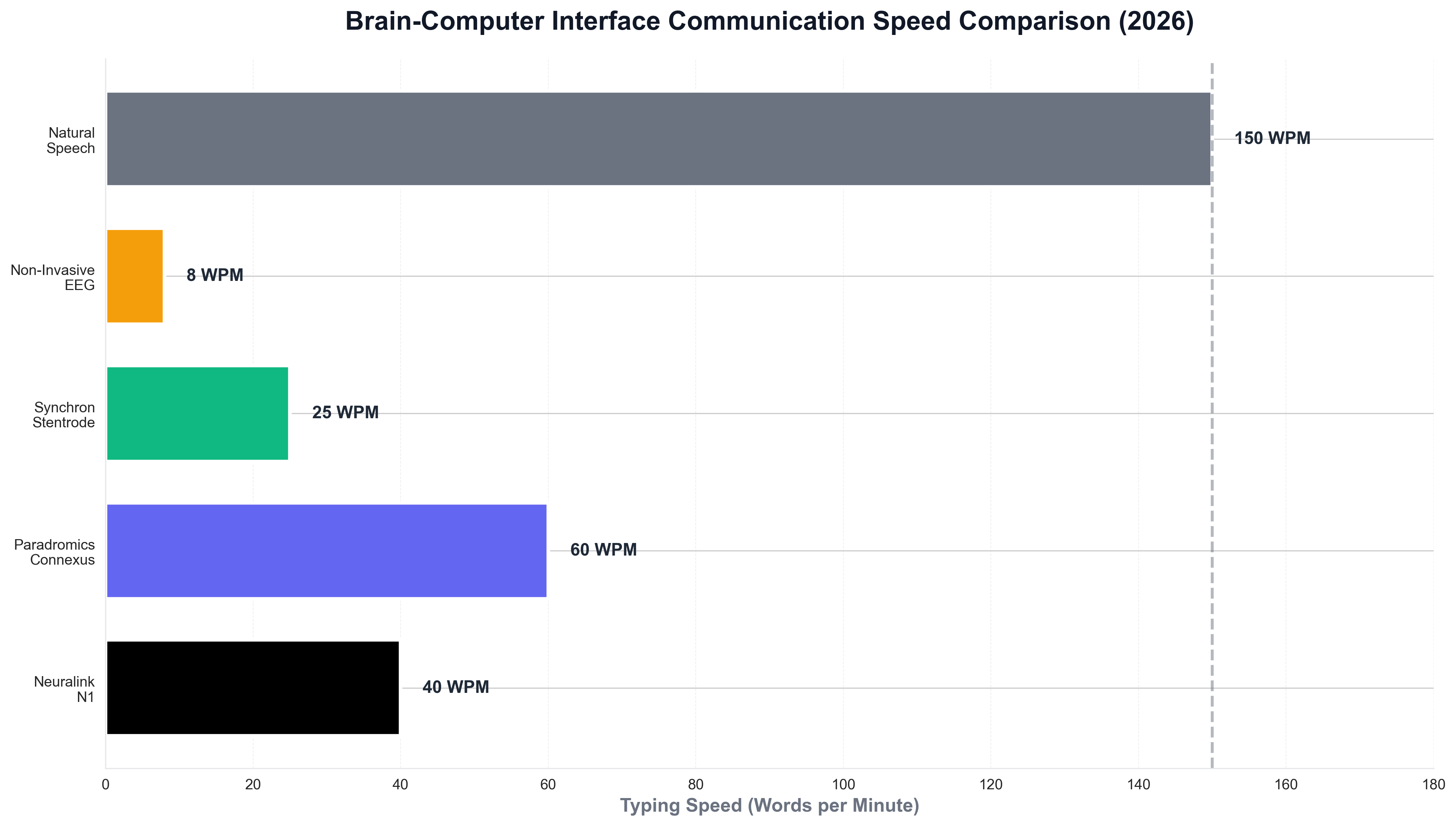
The typing speed comparison demonstrates the significant performance differences between BCI systems, with Paradromics achieving the highest speeds for communication applications while non-invasive systems offer lower performance but greater accessibility. The system has also enabled patients to control robotic arms with sufficient precision to perform tasks like eating and drinking independently, significantly improving quality of life.
The company is also developing applications for treating other neurological conditions, including blindness, deafness, and movement disorders. Neuralink's vision restoration program aims to create artificial vision by stimulating the visual cortex, while its hearing restoration program seeks to restore auditory function through direct neural stimulation. These applications represent ambitious goals that could benefit millions of patients worldwide, though they face significant technical challenges that must be overcome before they can become clinically viable.
Paradromics: High-Bandwidth Communication for Locked-In Syndrome
Paradromics has developed a specialized brain-computer interface optimized for high-bandwidth communication, targeting patients with locked-in syndrome who are conscious but unable to move or communicate. The company's Connexus system uses over 65,000 electrodes distributed across a flexible array that conforms to the brain's surface, enabling recording from a much larger area than traditional electrode arrays. According to Paradromics' clinical trial data, the system has enabled patients with locked-in syndrome to communicate at speeds approaching 60 words per minute, significantly faster than previous BCI systems.
The Connexus system's high electrode count enables it to record from a much larger number of neurons simultaneously, providing more comprehensive information about neural activity. This increased bandwidth is essential for communication applications, where speed and accuracy are critical for practical use. The system uses advanced signal processing algorithms to decode neural activity into text or speech, enabling patients to communicate naturally despite being unable to move or speak.
According to research published by Paradromics, the system has demonstrated the ability to decode neural activity with over 95% accuracy for communication tasks, enabling reliable communication for patients who were previously unable to express themselves. The system's high bandwidth also enables it to decode more complex neural patterns, potentially enabling control of more sophisticated devices or applications in the future.
Paradromics' approach focuses specifically on communication applications, optimizing the system for speed and accuracy rather than attempting to address the full range of BCI applications. This specialization allows the company to achieve better performance for communication tasks than more general-purpose BCI systems, though it limits the system's applicability to other uses. The company's success in communication applications demonstrates the value of specialized BCI systems designed for specific use cases.
Synchron: Minimally Invasive BCI Through Blood Vessels
Synchron has developed a fundamentally different approach to brain-computer interfaces, deploying electrodes through blood vessels rather than requiring invasive brain surgery. The company's Stentrode system is inserted through a blood vessel in the neck and navigated to the brain's motor cortex, where it expands to make contact with neural tissue. This approach eliminates the need for open brain surgery, reducing risks and making BCI technology accessible to a broader range of patients.
According to Synchron's clinical trial results, the Stentrode system has been successfully implanted in over 50 patients with paralysis, enabling them to control computers and communicate using only their thoughts. The system's minimally invasive approach has resulted in significantly lower complication rates compared to traditional brain implants, with no serious adverse events reported in clinical trials. This safety profile makes the system suitable for patients who might not be candidates for more invasive BCI procedures.
The Stentrode system's electrode count is lower than Neuralink's or Paradromics' systems, with 16 electrodes compared to thousands in other systems. However, the system's placement in the motor cortex and its stable contact with neural tissue enable it to achieve good performance for basic control tasks despite the lower electrode count. According to Synchron's published research, patients using the Stentrode system have achieved typing speeds of 20-30 words per minute, sufficient for practical communication while benefiting from the system's lower risk profile.
Synchron's approach represents an important alternative to more invasive BCI systems, potentially making brain-computer interfaces accessible to patients who cannot undergo brain surgery or prefer less invasive options. The company's success demonstrates that effective BCI systems don't necessarily require the highest electrode counts, and that less invasive approaches can provide meaningful benefits while reducing risks. This approach could expand the market for BCI technology by making it available to a broader range of patients.
Medical Applications: Restoring Lost Functions
Brain-computer interfaces are being used to treat a wide range of neurological conditions, with the most significant applications in paralysis treatment, communication restoration, and vision assistance. According to analysis from the National Institute of Neurological Disorders and Stroke, over 2,000 patients worldwide are now using BCI systems to restore lost functions, with the number growing rapidly as more systems receive regulatory approval and become commercially available.
Paralysis treatment represents the largest application area for BCIs, with systems enabling patients with spinal cord injuries, stroke, or other conditions to control computers, robotic prosthetics, and other devices. According to clinical trial data from multiple BCI companies, patients using BCI systems have achieved significant improvements in independence and quality of life, with many regaining the ability to perform daily tasks that were previously impossible. The systems enable patients to control wheelchairs, robotic arms, and computer interfaces, providing a level of independence that wasn't possible before.
Communication restoration is another major application, with BCI systems enabling patients with locked-in syndrome, ALS, or other conditions to communicate despite being unable to speak or move. According to research from BCI communication studies, patients using BCI systems for communication report significant improvements in quality of life, as the ability to express thoughts and needs is essential for human dignity and social connection. The systems enable patients to communicate with family members, caregivers, and healthcare providers, maintaining social connections that are critical for mental health and well-being.
Vision assistance represents a newer but promising application area, with BCI systems being developed to restore vision in blind patients by stimulating the visual cortex. According to research from vision restoration programs, early clinical trials have demonstrated that BCI systems can create basic visual perceptions in blind patients, though the quality and resolution of artificial vision are still limited compared to natural vision. These systems represent a significant advance over previous approaches to vision restoration, potentially benefiting millions of blind patients worldwide.
Non-Invasive BCIs: Expanding Access Through EEG and fNIRS
While invasive BCIs offer the highest performance, non-invasive brain-computer interfaces using electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) are expanding access to BCI technology for applications that don't require the highest performance levels. These systems don't require surgery, making them accessible to a much broader range of users, though they offer lower resolution and bandwidth compared to invasive systems.
According to research from non-invasive BCI developers, EEG-based systems can achieve typing speeds of 5-10 words per minute for communication applications, sufficient for basic communication needs while avoiding the risks and costs of brain surgery.
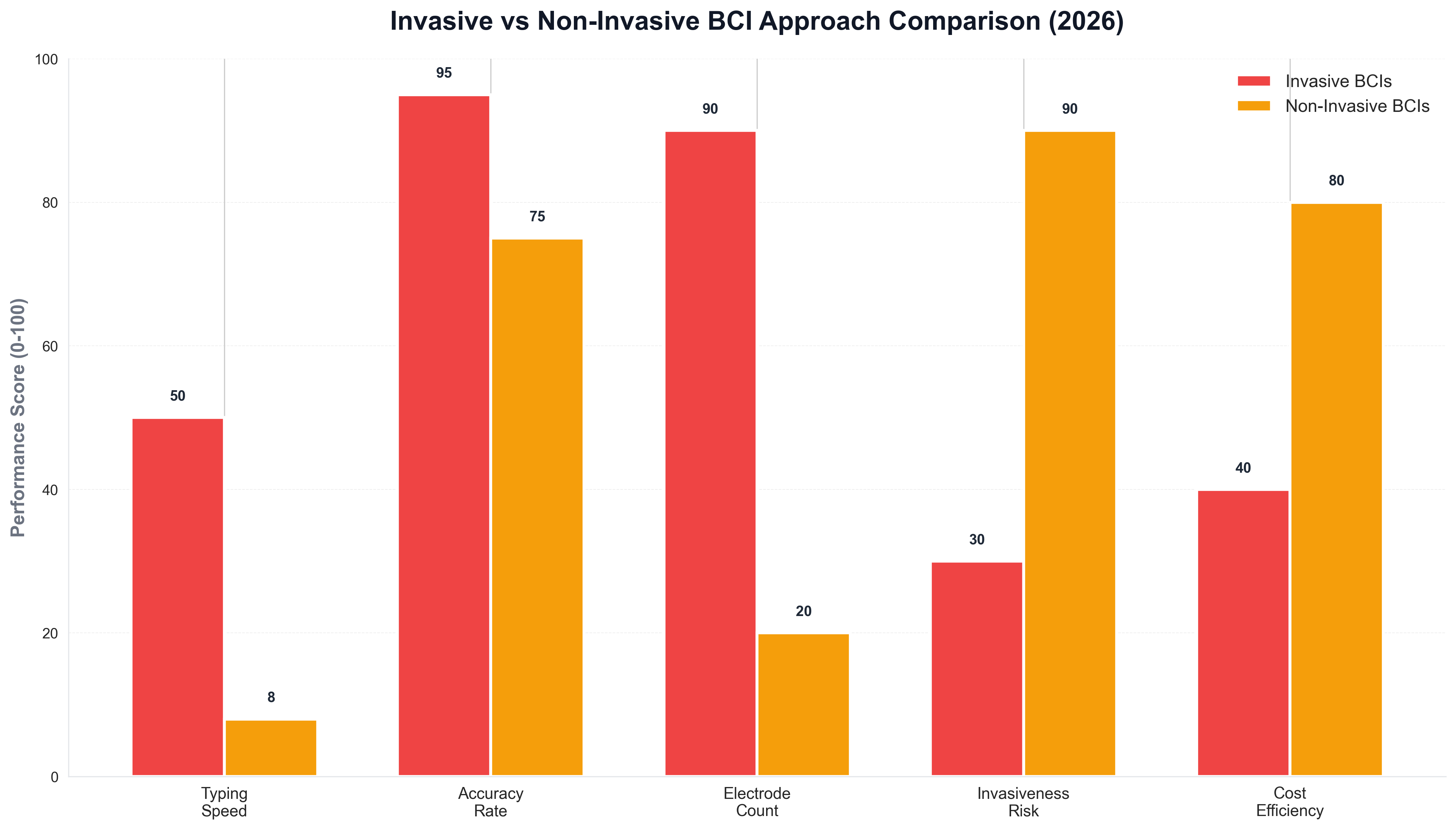
The approach comparison chart illustrates the trade-offs between invasive and non-invasive BCIs, with invasive systems offering superior performance in speed and accuracy while non-invasive systems provide better safety and accessibility, enabling patients to choose the approach that best matches their needs and preferences. These systems are being used for applications including assistive communication, gaming, and research, where the lower performance is acceptable given the benefits of non-invasiveness.
fNIRS-based systems offer intermediate performance between EEG and invasive BCIs, using near-infrared light to measure brain activity through the skull. According to fNIRS BCI research, these systems can achieve better performance than EEG for some applications while remaining non-invasive, making them suitable for users who need better performance than EEG but cannot or prefer not to undergo brain surgery. The systems are being developed for applications including stroke rehabilitation, cognitive training, and assistive communication.
The development of non-invasive BCIs is important for expanding access to brain-computer interface technology, as many potential users cannot or prefer not to undergo brain surgery. While these systems offer lower performance than invasive BCIs, they provide meaningful benefits for appropriate applications and enable BCI technology to reach a much larger user base. The continued improvement of non-invasive BCI technology could eventually narrow the performance gap with invasive systems, though significant technical challenges remain.
Safety and Regulatory Considerations
The safety of brain-computer interfaces is a critical concern, as these systems involve direct interaction with the brain, one of the most sensitive and important organs in the human body. According to safety analysis from the FDA, BCI systems have demonstrated generally good safety profiles in clinical trials, with serious adverse events occurring in less than 5% of patients. However, the long-term safety of these systems is still being evaluated, as many patients have only been using the devices for a few years.
The primary safety concerns with invasive BCIs include infection, inflammation, electrode failure, and long-term tissue damage. According to long-term safety studies, most BCI systems maintain stable performance for 2-5 years before requiring replacement or experiencing significant degradation. The risk of infection is managed through careful surgical procedures, sterile implantation techniques, and ongoing monitoring, though it remains a concern for long-term BCI use.
Regulatory approval processes for BCI systems are rigorous, requiring extensive clinical trials demonstrating both safety and efficacy. According to FDA approval data, the approval process typically takes 5-7 years from initial clinical trials to final approval, reflecting the complexity of evaluating brain-computer interfaces and the importance of ensuring patient safety. The regulatory framework continues to evolve as more BCI systems are developed and deployed, with agencies working to balance safety requirements with the need to enable innovation.
Ethical considerations are also important for BCI technology, as these systems raise questions about privacy, autonomy, and the nature of human identity. The ability to read and potentially influence neural activity creates concerns about privacy and data security, as neural data could reveal sensitive information about thoughts, emotions, and mental states. The potential for cognitive enhancement or modification raises questions about fairness, autonomy, and what it means to be human. These ethical considerations are being addressed by researchers, ethicists, and regulatory agencies as BCI technology continues to develop.
The Competitive Landscape: Multiple Approaches to Neural Interfaces
The brain-computer interface market has become increasingly competitive, with multiple companies developing different approaches to neural interfaces. Neuralink leads in high-density electrode arrays and has achieved the highest electrode counts, while Paradromics focuses on high-bandwidth communication applications. Synchron offers a less invasive alternative through blood vessel deployment, while companies like Blackrock Neurotech provide established systems with proven track records.
According to market analysis from BCI industry research, the global BCI market is expected to reach over $5 billion by 2030, driven by increasing regulatory approvals, improving technology performance, and growing awareness of BCI applications. The market includes both medical applications, which represent the majority of current revenue, and emerging consumer applications, which could become significant as technology improves and costs decrease.
The diversity of approaches in the BCI market is beneficial for patients, as different systems are optimized for different applications and patient needs. Some patients may benefit from high-performance invasive systems, while others may prefer less invasive options with lower performance. The competitive market is driving innovation and cost reduction, making BCI technology more accessible and effective for a broader range of patients.
Research institutions and universities are also playing important roles in BCI development, conducting fundamental research that informs commercial development and developing open-source systems that enable broader research and development. The combination of commercial development and academic research is accelerating progress in BCI technology, with innovations from research institutions often being adopted by commercial companies and vice versa.
Future Directions: Cognitive Enhancement and Human-Computer Integration
The future of brain-computer interfaces promises even more significant capabilities as technology continues to improve and new applications emerge. While current BCI systems focus primarily on medical applications, future systems could enable cognitive enhancement, direct brain-to-brain communication, and new forms of human-computer interaction. These applications raise important ethical and safety questions that must be addressed as the technology develops.
According to forecasts from BCI researchers, future BCI systems could enable direct thought-to-text communication at speeds approaching natural speech, memory enhancement through neural stimulation, and direct brain-to-brain communication for sharing thoughts and experiences. These capabilities could transform how humans interact with computers and each other, though they also raise significant ethical concerns about privacy, autonomy, and human identity.
The development of bidirectional BCIs—systems that can both read from and write to the brain—could enable even more sophisticated applications, including sensory restoration, memory enhancement, and cognitive augmentation. However, these capabilities also raise concerns about safety, as writing to the brain could potentially cause unintended effects or be used for harmful purposes. The development of bidirectional BCIs will require careful research, rigorous safety testing, and thoughtful ethical consideration.
The integration of BCI technology with artificial intelligence could also create new possibilities, as AI systems could interpret neural signals more accurately, predict user intentions, and adapt to individual users' neural patterns. This integration could improve BCI performance while also raising questions about privacy, as AI systems would have access to detailed neural data. The development of AI-enhanced BCIs represents an important area of research that could significantly improve the technology's capabilities.
Conclusion: Brain-Computer Interfaces as a Medical and Technological Revolution
Brain-computer interfaces have reached a transformative moment in 2026, with multiple systems receiving regulatory approval and successfully treating thousands of patients worldwide. The technology's ability to restore lost functions, enable communication, and potentially enhance human capabilities represents one of the most significant developments in medical technology and human-computer interaction. As BCI systems continue to improve and become more accessible, they will play an increasingly important role in treating neurological conditions and potentially expanding human capabilities.
The competitive market in brain-computer interfaces is driving innovation and improving patient outcomes, with different companies developing specialized systems for different applications. This diversity benefits patients by providing options that match their specific needs and preferences, while competition drives cost reduction and performance improvement. As the BCI market continues to grow, it will enable more patients to benefit from these transformative technologies.
The ethical and safety considerations surrounding BCI technology are important and must be addressed thoughtfully as the technology develops. The ability to read and potentially influence neural activity creates significant responsibilities for developers, regulators, and users. However, the potential benefits of BCI technology—restoring lost functions, treating neurological conditions, and potentially enhancing human capabilities—are substantial and justify continued development with appropriate safeguards.
As we look toward the future, brain-computer interfaces will continue to evolve, becoming more capable, safer, and more accessible. The technology's potential to transform medical treatment, human-computer interaction, and even human capabilities makes it one of the most important technological developments of our time. Brain-computer interfaces are not just a medical technology—they represent a fundamental shift in how humans interact with technology and potentially how we understand and enhance human capabilities.




